
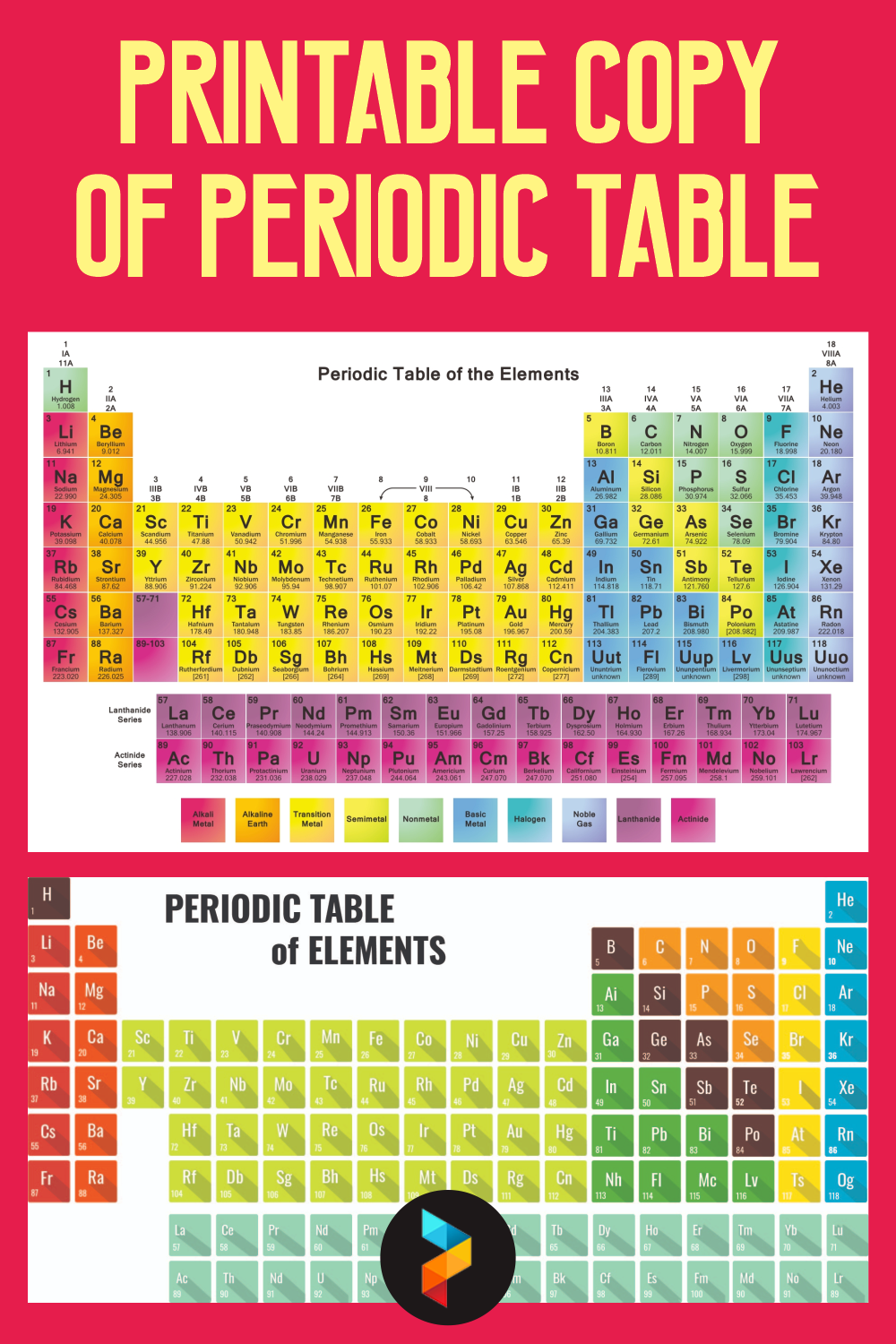
In all other cases, the value is the mean atomic weight of the most stable isotopic composition, according to Atomic Weights of the Elements 2001, and includes its uncertainty in parenthesis. † A value in brackets, such as, is the atomic weight of the most stable isotope unless it is an integer, in which case it is the mass number of the most stable isotope. State at standard temperature and pressure (0☌ and 1 atm) Gases

Group →Ĭhemical series of the periodic table Alkali metals The key below explains the colour-coding and layout of each entry.

The large version of the periodic table set out below does not fit into some computer screens however, with a small font size and/or in landscape mode, it may be possible to print this periodic table on one or two normal-size sheets of paper.


 0 kommentar(er)
0 kommentar(er)
